Buy JWH-018 fertilizer online | Bonsai Tree Fertilizer JWH-018 for sale 2024-25 If you are looking for the best JWH-018 fertilizer to buy that will help your plants grow better in your garden, then you have come to the right place. RCChemSupply is a one-stop online store that provides the best quality Jwh-018 Fertilizer at […]
Tag Archives: How to make jwh 018 recipe
Guide to Creating a JWH-018 Recipe
Welcome to our comprehensive guide on creating a JWH-018 recipe. At rcchemsupply.net, we strive to provide you with the most reliable and accurate information to help you navigate the world of research chemicals.
Understanding JWH-018
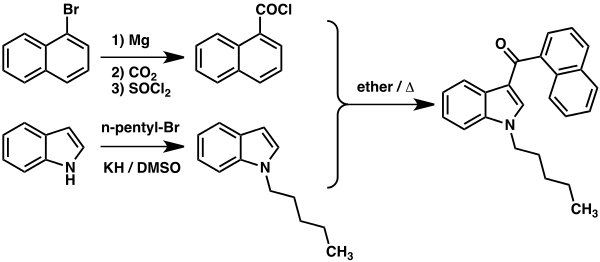
JWH-018, also known as 1-pentyl-3-(1-naphthoyl)indole, is a synthetic cannabinoid that acts as a potent agonist of the CB1 receptor. It was first synthesized by Dr. John W. Huffman in 1995 and gained popularity in the research community due to its structural similarity to THC, the primary psychoactive compound found in cannabis.
Legal Status and Regulations
The legal status of JWH-018 varies across different countries. It is important to note that the information provided in this article is for educational purposes only and should not be considered legal advice. In some countries, JWH-018 is classified as a controlled substance and is illegal to possess, sell, or distribute. It is crucial to familiarize yourself with the laws and regulations regarding synthetic cannabinoids in your jurisdiction [1].
Chemical Composition and Structure
JWH-018 has a chemical formula of C24H23NO and a molar mass of 341 g/mol. Its IUPAC name is (Naphthalen-1-yl)(1-pentyl-1H-indol-3-yl)methanone. The compound consists of a naphthalene ring and an indole ring, which are responsible for its cannabinoid-like effects [1].
Synthesis of JWH-018
The synthesis of JWH-018 involves several chemical reactions, which we will outline below.
1. Precursor Chemicals
The starting materials for JWH-018 synthesis include naphthalene, 1-pentylindole, and 1-pentanoyl chloride. These precursor chemicals provide the necessary building blocks for the formation of the desired compound.
2. Acylation Reaction
The first step in the synthesis process is the acylation reaction. In this step, 1-pentylindole is reacted with 1-pentanoyl chloride to form the intermediate compound, 1-pentyl-1H-indol-3-ylpentan-1-one. This reaction is typically carried out in the presence of a base and a suitable solvent.
3. Cyclization
The next crucial step is the cyclization of the intermediate compound. Under specific reaction conditions, the ketone group in the intermediate compound reacts with naphthalene, leading to the formation of JWH-018. This cyclization reaction is often facilitated by the use of Lewis acids or other catalysts.
4. Purification and Analysis
Once the synthesis is complete, the resulting product, JWH-018, undergoes purification to remove any impurities or byproducts. Various purification techniques such as chromatography or recrystallization can be employed to obtain a pure form of JWH-018. The purity of the compound is crucial for both research purposes and safety considerations.[2][7][9].
Safety Disclaimer
Before we proceed, it is crucial to emphasize that the synthesis and use of JWH-018 or any other research chemical should only be conducted by qualified individuals in a controlled laboratory environment. Engaging in any illegal activities or misuse of chemicals is strictly prohibited and can have severe legal and health consequences.
Ingredients and Equipment
To initiate the synthesis process, you will need the following ingredients and equipment:
Ingredients:
- Naphthalene
- 1-Aminoalkanes
- Acid chloride
- Base
- Organic solvents (e.g., toluene, ethanol)
Equipment:
- Round-bottom flask
- Condenser
- Separatory funnel
- Glass stirring rod
- Heating mantle
- Thermometer
Synthesis Process
Please note that the synthesis process described here is for educational purposes only. We strongly advise against attempting to create JWH-018 without proper authorization and expertise.
Step 1: Preparation
- Set up a well-ventilated laboratory workspace and ensure you have all the necessary safety equipment, such as gloves and goggles.
- Weigh the appropriate amounts of naphthalene, 1-aminoalkanes, and acid chloride as per your desired recipe. These quantities may vary based on the specific synthesis procedure you choose to follow.
Step 2: Reaction
- Dissolve naphthalene in an organic solvent, such as toluene, in a round-bottom flask.
- Add the acid chloride slowly to the flask, stirring the mixture gently with a glass stirring rod.
- Maintain a controlled temperature using a heating mantle, and continue stirring until the reaction progresses. The exact temperature and duration may depend on the specific synthetic pathway you are following. It is crucial to consult reputable literature or an expert in the field for precise guidelines.
- During this process, be cautious of the reaction’s exothermic nature, and monitor the temperature carefully.
Step 3: Workup
- Once the reaction is complete, allow the mixture to cool down to room temperature.
- Transfer the reaction mixture to a separatory funnel, carefully separating the organic and aqueous layers.
- Extract the organic layer containing the synthesized JWH-018 using an appropriate organic solvent, such as ethanol.
- After extraction, you can evaporate the solvent to obtain the final product, which may require further purification steps depending on your desired level of purity.
Safety Considerations
When handling and synthesizing research chemicals like JWH-018, it is crucial to prioritize safety. Here are some essential safety considerations to keep in mind:
Proper Laboratory Setup
- Ensure you have a well-ventilated workspace with adequate fume hoods to prevent the buildup of harmful vapors.
- Use proper personal protective equipment, including gloves, lab coats, and goggles.
- Familiarize yourself with emergency procedures and have appropriate safety equipment readily available, such as fire extinguishers and spill containment materials.
Chemical Handling
- Handle all chemicals with care and in accordance with their safety data sheets (SDS).
- Follow proper storage guidelines to prevent accidental mixing or exposure to incompatible substances.
- Dispose of chemical waste responsibly and in compliance with local regulations.
Conclusion
While the synthesis of JWH-018 may be of interest to researchers in the field, it is essential to approach it with the utmost caution and respect for legal and safety considerations. At Rcchemsupply.net, we aim to provide valuable information to the research community while emphasizing the importance of responsible chemical use. Always consult with experts, adhere to legal regulations, and prioritize safety when conducting any research involving JWH-018 Recipe or other research chemicals.
Diagram: A simplified representation of the JWH-018 synthesis process.
Please note that this article is purely informational and should not be interpreted as an endorsement or encouragement to engage in any illegal activities. The synthesis of JWH-018 should only be pursued in compliance with applicable laws and regulations and with proper authorization from relevant authorities.
For more in-depth information on research chemicals, their synthesis, and responsible use, feel free to explore our website and consult with experts in the field.
What is JWH-018? |JWH 018 for sale | Everything you need to know about JWH-018 what is JWH-018? JWH 018 powder is a synthetic cannabinoid. An organic chemist named John W. Huffman first made it in 2008. It gained popularity in 2009. German chemists found it then. It was a chemical in the popular synthetic […]